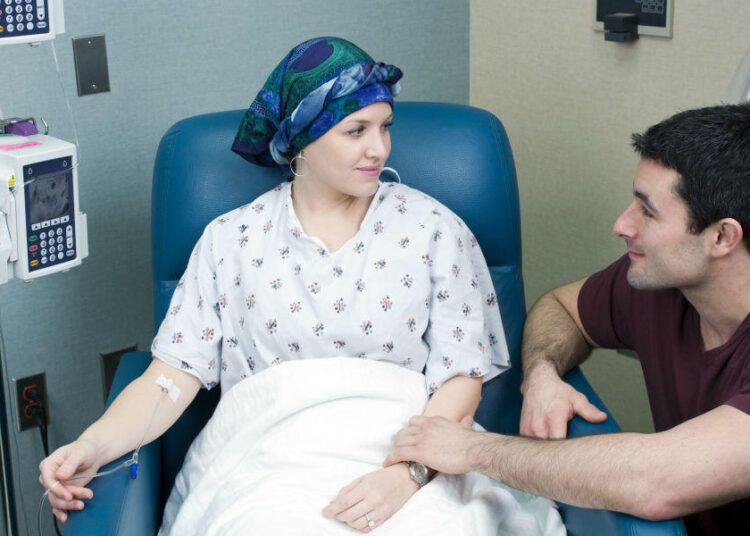
Hundreds of blood cancer patients in England are set to benefit from an innovative new treatment after the NHS approved lisocabtagene maraleucel, also known as liso-cel or Breyanzi. The treatment, developed by Bristol Myers Squibb, was recently given the green light by the National Institute for Health and Care Excellence (NICE).
Liso-cel is a form of CAR T-cell therapy that uses a patient’s own immune cells to fight cancer. The process involves extracting T-cells from the patient’s body, genetically modifying them in a lab to specifically target cancer cells, and then reinfusing them back as a one-time, personalized treatment. It has been recommended for patients with large B-cell lymphoma, an aggressive type of blood cancer, particularly for those whose disease has not responded to initial treatments or has relapsed within a year.
Liso-cel: A breakthrough in blood cancer treatment
According to NICE, the approval of liso-cel is expected to benefit approximately 600 patients annually. Clinical trials have shown that the therapy significantly slows disease progression, with patients living more than twice as long without worsening symptoms compared to those receiving standard treatments.
Helen Knight, director of medicines evaluation at NICE, emphasized the impact of this approval, stating: “For people living with this aggressive blood cancer, and their families, today’s announcement offers real hope. These aren’t just statistics—each person who will benefit is someone’s parent, child, partner, or friend.”
Cancer Research UK estimates that around 5,000 people are diagnosed with large B-cell lymphoma in the UK each year. The introduction of liso-cel provides a much-needed alternative to traditional treatments, many of which come with severe side effects and limited effectiveness.
NHS reverses initial decision, prioritizing patient care
Initially, the NHS had decided against offering this treatment due to its high cost, with a single infusion priced at £297,000. However, following negotiations with Bristol Myers Squibb, an improved commercial agreement was reached, making the therapy accessible to patients in England.
Professor Peter Johnson, national director for cancer at NHS England, welcomed the decision, saying: “The NHS remains dedicated to funding the best cancer care, and it is fantastic that we can offer treatments like this to hundreds more patients with advanced blood cancers. This provides real hope for a longer and better quality of life.”
Advancing cancer treatment and accessibility
The approval of liso-cel marks a significant milestone in cancer treatment advancements, as it becomes the fourth CAR T-cell therapy available for NHS patients. Since the introduction of these therapies, over 1,500 individuals have received life-extending treatment through the NHS.
Josh Hill, policy officer at Blood Cancer UK, also praised the decision, highlighting the urgent need for better treatment options. “Blood cancer is the UK’s third-biggest cancer killer. Many of the existing treatments are highly toxic, so we welcome this new option that provides a better alternative. Continued research and investment are crucial to ensuring that all patients across the UK have access to life-saving treatments,” he stated.
As the NHS continues to improve access to groundbreaking therapies, the approval of liso-cel represents a crucial step toward enhancing survival rates and the quality of life for blood cancer patients.








 India
India